Infrastructure
- Bion Clinicals
- Infrastructure

Infrastructure
Screening Area
- Dedicated Screening area spread across 3,000 Sqft. with ample space for volunteer waiting and recreation.
- 2 ICF Rooms
- 2 ECG Rooms
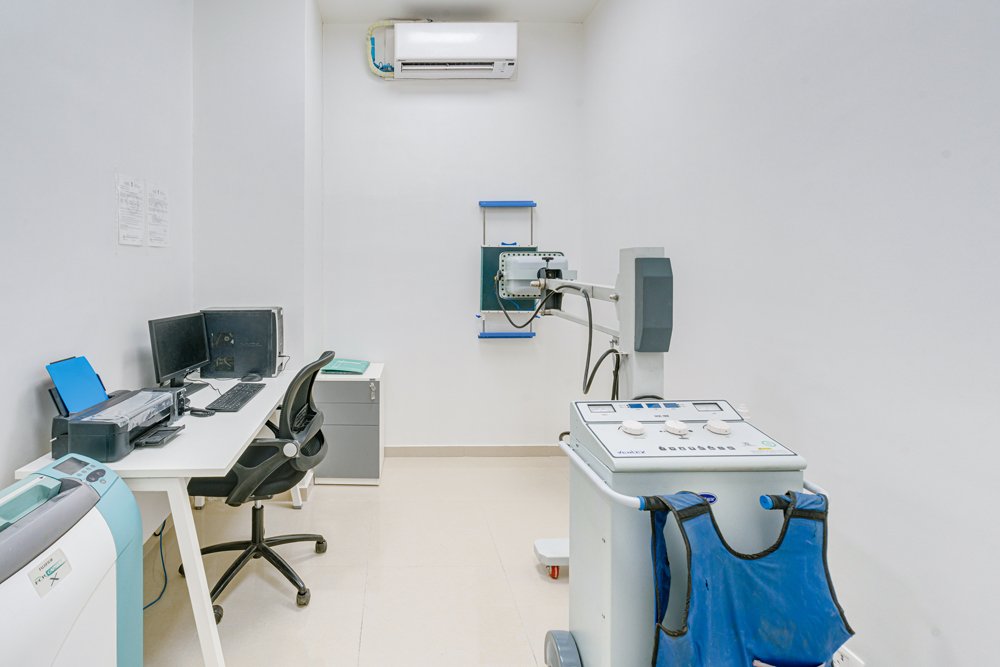
- 2 Medical Examination Rooms.
- In-house X-Ray Unit
- Allocated Screening Phlebotomy area with 2 Dry
- Assigned Study Presentation area
Clinical Pharmacological Unit (CPU)
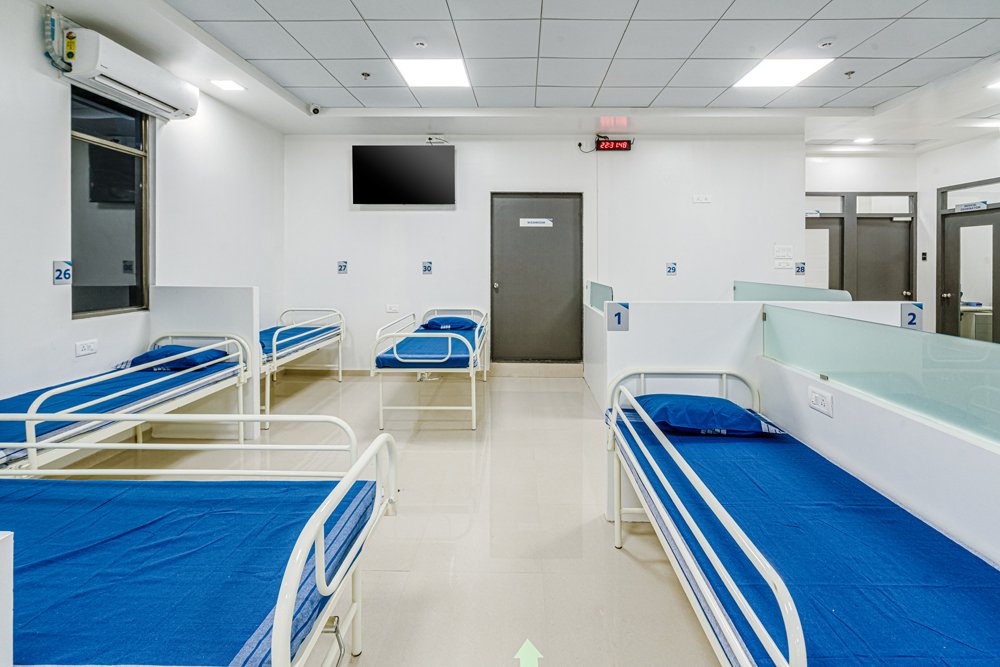
- 2 CPU with 30 beds capacity each, supported with 3 Bedded fully functional modern ICU.
- Dedicated Dosing and Phlebotomy area
- Allocated sample processing and storage area equipped with -80°C Degrees Secure Deep Freezers and refrigerated centrifuge machine
- Both CPUs are equipped with Monochromatic light for light sensitive molecules
- Dedicated Pantry and Recreational area
BioAnalytical Area
Bion Clinical involves testing for drug concentrations, metabolites, and biomarkers using advanced techniques. Bioanalytical services are crucial for pharmacokinetics (PK), pharmacodynamics (PD), and toxicology studies. This area ensures that regulatory requirements are met and provides essential data for decision-making in drug development.
Facility at Glance
Collaboration is at the heart of what we do. We work closely with our sponsors , regulatory agencies, and stakeholders to ensure every BABE studies are conducted with the highest standards of ethical integrity and efficiency. Through our passion for science, combined with our dedication to subject safety, we strive to create meaningful impacts in the clinical research industry. As we continue to evolve and expand, our commitment to innovation, excellence, and service remains unwavering. Sponsor seeking a trusted BABE CRO/research partner or a patient Based studies/ Clinical trials we are here to assist you every step of the way.